By Alec Chaves
Technique Name: Radioactive Tracing in Metabolic Research
Danger Rating: ![]()
As the name suggests, this technique requires the scientist to handle radioactive materials such as tridium and 14-carbon, and if they do not wear protective equipment (gloves, eyewear, lab coat) and/or use unsafe lab practices, they could be accidentally exposed to the radiation which could result in injuries anywhere from minor burns to fatal radiation sickness.
What is the general purpose? This technique allows scientists to track how nutrients (fats, proteins, sugars, minerals, vitamins) are consumed and processed by cells and tissues to use for energy.
Why do we use it? Diseases like diabetes and obesity have been shown to result from changes in how tissues and cells metabolize nutrients. Radioactive tracers are tools that are used to determine what these changes are and how certain lifestyle modifications and medications can fix these differences.
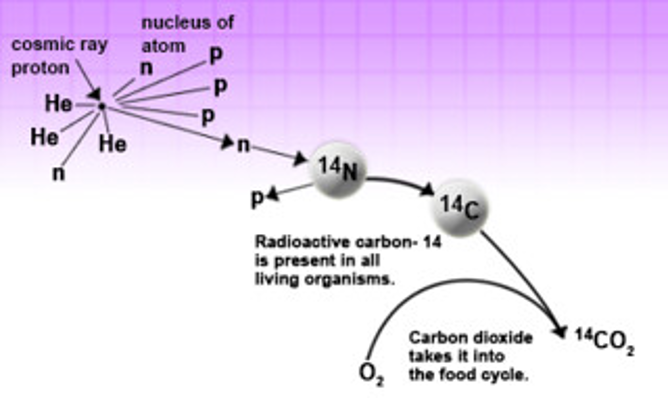
Example of nuclear fusion to create radioactive carbon atoms. When a nitrogen atom absorbs neutrons, they release protons from their nucleus, resembling a carbon atom, which have 12 protons and 14 neutrons.
How does it work? Before I go into the technique, it is important to discuss the chemistry behind radioactive tracers. As you probably learned in class, all matter is made of atoms. The core of an atom, or the nucleus, consists of protons and neutrons. Most nuclei have an equal number of protons and neutrons. However, natural processes, such as supernovas, produce astronomical amounts of energy, forcing nuclei to fuse with one another, creating a new atom as shown above.
Some of these new atoms have a higher number of neutrons than protons. Neutrons are very unstable when they are not paired with a proton which causes the nucleus to start breaking down, or decaying. As the atoms break down, energy is released in the form of radiation or radioactivity. Luckily for us, natural radioactive atoms are very rare and therefore do not pose a threat to us. However, scientists have developed ways to artificially produce these atoms as well as other substances made up entirely of radioactive atoms. For instance, the lab I work in regularly uses sugar molecules that contain radioactive carbon atoms. Why, you might ask?
Well, sugar is a nutrient we consume during essentially every meal and is an important fuel source for all the cells in our body. However, in individuals with diabetes, some of their tissues lose the ability to take in sugar and use it for energy. Therefore, it will continue to float around in their bloodstream, eventually leading to high blood sugar levels, which can become extremely toxic. In order to fix this, scientists need to determine ways to increase the rate at which cells take up the sugar in the bloodstream.
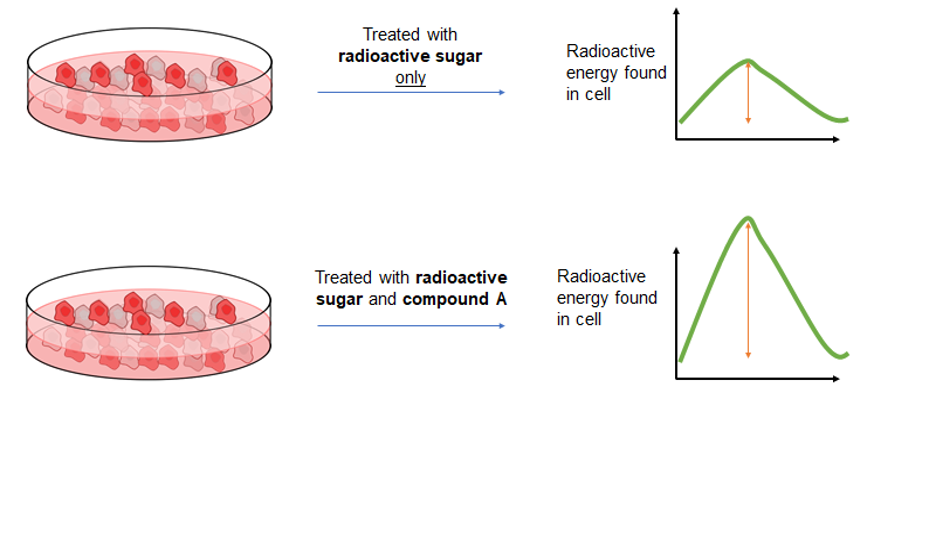
Example of an experiment to determine if “compound A” increases the rate of sugar uptake into cells.
In case you were worried about where I was going with this, giving radioactive sugar is not the cure to diabetes and is not given to the individual directly. Instead, scientists obtain cells from these individuals and grow them separately in a petri dish. Once these cells are mature enough, the scientists can then treat the cells with radioactive sugar. As I mentioned above, radioactive materials give off energy as they are breaking down. Therefore, once the radioactive sugars are placed on the cells, they can track how much of it is absorbed based on the amount of radioactive energy found in the cell once the experiment is complete. The figure above details an example of an experiment to determine if “compound A” increases the rate at which sugar is taken up. Both sets of cells are treated with radioactive sugar, however, only the cells on the bottom are treated with compound A. Once the experiment is complete and the cells are collected, the amount of radioactivity in the cells is measured. As you can see, the height of the curve is bigger for cells treated with compound A. This means there is a greater amount of sugar in the cells treated with compound A, so we can conclude that compound A increases the rate at which these cells take up sugar. This tracing concept can be extended to other nutrients as well. Not only that, but there are ways to figure out how much of the tracer is stored away for later use in a cell, or broken down to be used for immediate energy production.
