By Rami Major
The longest gene in humans is called DMD, which facilitates the production of a protein called dystrophin. Dystrophin plays a role in strengthening both skeletal and cardiac muscle fibers. It is largely responsible for your ability to move and pump blood throughout your body. The DMD gene is considered “X-linked” because it is located on the X chromosome. When mutated, it can lead to a disease called Duchenne’s muscular dystrophy, a severe disease that leads to muscle wasting and heart problems. Duchenne’s muscular dystrophy is an X-linked recessive disease. This means that if you have a normal DMD allele, or normal copy of the DMD gene, you will not have the disease. For example, in females who have two X chromosomes, the DMD allele would have to be mutated on BOTH chromosomes to cause disease, unlike in men who have only one X chromosome and one Y chromosome, meaning a mutation in the DMD gene would automatically cause Duchenne’s muscular dystrophy in this case. This makes Duchenne’s muscular dystrophy much more common in males, who only have one X chromosome. If their single copy of DMD is mutated, they will develop the disease.

Golden retrievers are an animal that are also susceptible to a form of DMD, termed Golden Retriever Muscular Dystrophy (GRMD). The above dog is healthy, but dogs with GRMD experience many of the same symptoms as humans with DMD, including muscular atrophy. Norsk bokmål, Wikimedia Commons.
Currently, most treatments for Duchenne’s muscular dystrophy are palliative, meaning they will not cure or fix the disease but can only provide relief or comfort from symptoms. However, the underlying issue–lack of dystrophin–is not addressed. Golden Retrievers have a similar form of this disease, dubbed Golden Retriever muscular dystrophy (GRMD). In 2017, a company called Sarepta Therapeutics developed a gene therapy which restored muscle function in Golden Retrievers with the disease. Mutations in the DMD gene can result in a type of mutation called a frameshift mutation, causing a nonfunctional dystrophin protein to be synthesized. The gene therapy that has been developed takes a shortened but working form of the dystrophin protein, packages it in a viral vector, and delivers it to the diseased dogs. In this video, you can see dogs afflicted with GRMD who have received the gene therapy treatment hopping over small obstacles and wagging their tails, feats that would’ve been considered impossible before this treatment was available.
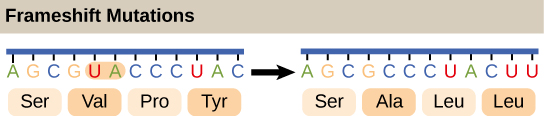
Frameshift mutations, or mutations that cause the reading frame to shift and a different protein to be produced, are responsible for diseases like DMD. Openstax Biology, Wikimedia Commons.
While Sarepta Therapeutics was testing their gene therapy in dogs, Jude Samulski, a scientific researcher at the University of North Carolina and leader in the gene therapy field, was testing a Duchenne’s muscular dystrophy gene therapy of his own. He tested this first in mice, then in dogs, and eventually secured approval for a clinical trial to test the gene therapy in humans. Much like the therapy developed by Sarepta, this therapy used a smaller version of the dystrophin gene, and delivered this to the diseased cells in the body using a viral vector. Samulski’s technology was upscaled by a major pharmaceutical company called Pfizer, and a boy named Conner Curran was the first to receive the novel treatment in early 2018. Recently, NPR reported that Conner went from being unable to walk up the stairs to running up them, suggesting that the gene therapy is succeeding for now.
Despite these successes, there are several concerns with gene therapy. Firstly, there are concerns that the viral nature of gene therapy delivery will affect the immune system, as patients who received the aforementioned gene therapy experienced firsthand symptoms such as fevers and kidney problems. Secondly, it is unknown how long the effects of gene therapy will last, and whether or not further treatment would be safe. Additionally, the long lasting effects of gene therapy are unknown. It is unclear whether or not further ailments would result due to this treatment. This is why clinical trials are so important–they help researchers study the treatment and its outcomes at great lengths before making it readily available.
Although gene therapy comes with risks, the stakes are high and the potential reward- a cure- is arguably worth it. Scientists are working hard to find a safe and lasting treatment for this debilitating disease, and the promising trajectory of the field is certainly exciting!
