By Regina Fernandez
Fun Rating:![]()
Difficulty Rating:![]() Image from Biorender
Image from Biorender
What is a lipid? Lipids are organic hydrophobic molecules that do not mix well with water. Some examples of lipids include fats, phospholipids, and cholesterol, which are found in food and in living organisms. Lipids in our body are used to produce and store energy, are building blocks for cell membranes, and are used to communicate with other cells.
What is the general purpose? The purpose of this technique is to separate lipids from other molecules in the cell such as proteins, sugar, or DNA. After extraction, scientists can identify the different types of lipids.
Why do we use it? There are many reasons why someone would like to extract lipids. For example:
- Lipids extracted from organisms like algae are used for the production of biodiesel, a renewable fuel.
- Lipids can affect the physical properties of foods. For example, a food product containing high amounts of saturated fat will make it more solid whether more unsaturated fat will make it more liquid. Food scientists can use this information to adjust the physical properties of foods.
- Companies that produce food are required to report the specific amount of each lipid and the type of lipid on their food labels. The type of lipids found in foods can have an effect on our health. Thus, knowing the type of lipids found in food products can keep us better informed so that we can make informed decisions about what we put in our bodies. Scientists can determine how diet, exercise, or other factors can alter the type and amount of lipids found in tissues and cells.

Figure 1.Car using biodiesel as fuel (Left) and food label showing the amount of fat found in that product (Right).
How does it work?
The Folch lipid extraction method was first described by Jordi Folch back in 1957 and is considered one of the gold-standard methods to extract lipids from biological samples. The technique takes advantage of the fact that many lipids are non-polar compounds that will mix well with non-polar solvents but not with water, which is polar. Using different types of solvents you can separate lipids from water-soluble compounds like proteins and carbohydrates. The Folch method requires the use of chloroform (a non-polar solvent), methanol (a polar solvent), and water. Because chloroform is toxic the procedure has to be performed under a fume hood and you should wear personal protective equipment (gloves, lab coat, goggles). Caution has to be taken when handling the solvents.
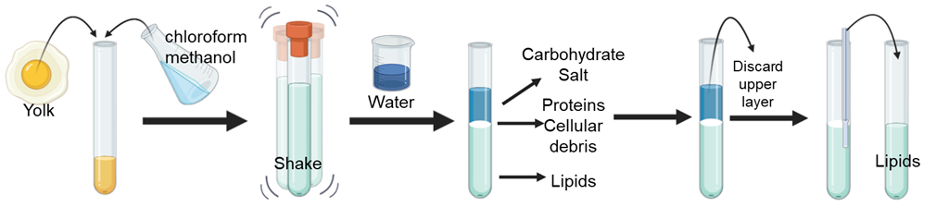
Figure 2. Lipid extraction technique. Image created by author with BioRender.com
Let’s pretend you want to quantify the amount of lipids found in an egg yolk. The first step is to mix in a tube the egg yolk with a solution made of chloroform and methanol. Next, you cap the tube and shake it for a couple of minutes. After shaking you should see a homogenous solution. Here is where the lipids will start to associate with the chloroform and the other water-soluble, polar components will associate with the methanol. Then, you add water to wash the solvent, shake for at least 30 seconds, and spin the tube using a centrifuge. The addition of water results in the generation of two layers, also known as phases which will be further separated during the centrifugation step according to their densities. One phase contains the non-polar lipids mixed with the chloroform and the other phase the water-soluble components of the egg yolk mixed with water and methanol. The chloroform phase will be found at the bottom of the tube and the methanol/water in the top because chloroform is more dense. Between the two layers, you will find a very thin solid layer, which will contain proteins and cellular waste. To recover the lipids, you first discard the upper layer. Using a pipette, carefully introduce the tip until it reaches the bottom and then transfer the lower phase to a clean tube. Try not to disturb the thin solid layer to prevent contamination of your lipid extracts. Congratulations! You just learned how to extract lipids from an egg yolk!
After lipid extraction scientists may evaporate the chloroform with nitrogen and store the lipids as dry extracts until they are ready to be analyzed. Over the years scientists have modified the Folch lipid extraction method. For example, different solvents can be used to extract specific types of lipids. Another reason is to reduce the use of hazardous solvents like chloroform and substitute it for more environmentally friendly solvents.
Note: Most lipids are non-polar, however there are a few that are polar. Thus, before scientists extract lipids they need to know which type of lipid they are interested in. That way they know in which phase they will find it or if they need to adjust the protocol.
