By Alan Curtis
As humans, we are constantly interacting with our environments: we sit on the couch, we ride in cars, we go for walks, we swim in rivers, lakes, and oceans, we (used to) get on planes. The number of ways we experience and manipulate our environment leaves us susceptible to injury and disease not only from foreign material (viruses, bacteria, fungi, allergens, etc.), but also our own proteins (autoimmunity). Thus, the immune system is charged with protecting us from a seemingly infinite universe of antigens (substances that the immune system can recognize). Therefore, the immune system must be able to distinguish self from non-self.
The immune system is often organized into two major systems: the innate and the adaptive systems. The innate immune system includes all of the physical and chemical defenses that we are born with: skin provides a physical barrier against the environment, tears contain enzymes that break down bacterial cell walls, our higher body temperature inhibits fungal growth, etc. The adaptive immune system, conversely, is shaped by our experiences. The adaptive immune system has a memory and can remember previous infections such as the chicken pox. It can also be programmed by vaccines to protect us from diseases like the flu, tetanus, Polio, measles and more.
The adaptive immune response is, on its simplest level, made up of two separate yet interdependent cell types: B lymphocytes and T lymphocytes (often just called “T cells”). In the 1970s and 1980s it was widely accepted that 3 subtypes of T cells existed. Today, there is evidence that up to 50 different types exist! Highly sophisticated experimentation with a laser-based approach (flow cytometry) has opened our eyes to the seemingly endless complexity and diversity of these immune cells.
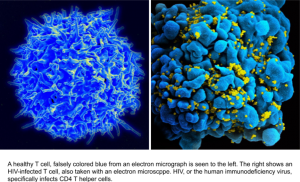
T cells were first discovered in 1968 when researchers found that both the thymus and bone marrow were required to produce robust antibody responses. For the past 60 years the T (for thymus) helper lymphocyte has been recognized as a large player in how the immune system works and the focus of intense study. So, what the heck are T cells and what do they do?
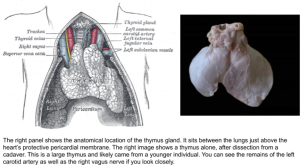
T cells, like all lymphocytes, begin as progenitor cells in the bone marrow. Chemical signals tell the would-be T cells to migrate to the thymus – a small gland in the neck that begins producing T cells during fetal development. Over time, the thymus slowly shrinks and decreases its output of T cells. By the time a person reaches 25, there is very little thymic activity. This is one of the reasons why elderly people are considered immunosuppressed – they are not making any new T cells and the ones made 40+ years ago are beginning to die (certain memory T cells can live for decades and respond over and over to repeat infections!).
 Once the progenitor T cell arrives at the thymus, a series of complex and random genetic mutations takes place. During this process, a T cell gains specificity by forming an antigen-specific receptor on its surface. T cell receptors are capable of recognizing protein pieces, called peptides, but cannot respond to fats, carbohydrates, or nucleic acids the way that antibodies (the B cell antigen-specific receptor) can. The T cell receptor is made up of two chains and crosses the cellular membrane of the T cell. Once the target is recognized by the T cell receptor, signals are sent inside the cell that ramp up the immune response. T cells reproduce through mitosis and generate an army of clones ready to take down the infection. This is why your lymph nodes (areas of concentrated immune cells) swell when you get sick – massive amounts of cell division! Don’t worry, though. After the infection is cleared, 99.9% of these T cells die off and the remaining 0.1% keep the memory of the infection safe for next time.
Once the progenitor T cell arrives at the thymus, a series of complex and random genetic mutations takes place. During this process, a T cell gains specificity by forming an antigen-specific receptor on its surface. T cell receptors are capable of recognizing protein pieces, called peptides, but cannot respond to fats, carbohydrates, or nucleic acids the way that antibodies (the B cell antigen-specific receptor) can. The T cell receptor is made up of two chains and crosses the cellular membrane of the T cell. Once the target is recognized by the T cell receptor, signals are sent inside the cell that ramp up the immune response. T cells reproduce through mitosis and generate an army of clones ready to take down the infection. This is why your lymph nodes (areas of concentrated immune cells) swell when you get sick – massive amounts of cell division! Don’t worry, though. After the infection is cleared, 99.9% of these T cells die off and the remaining 0.1% keep the memory of the infection safe for next time.
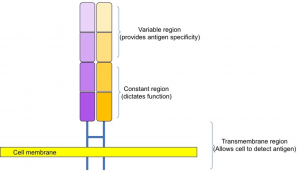
Here is a cartoon drawing of the T cell receptor. The arrangement is similar to the structure of an antibody but is bound to the cellular surface and is not Y-shaped. The variable region is the location of much genetic mutation during development of the T cell and will dictate its specificity to a particular protein. The constant region, along with the variable region, plays a role in restriction, as discussed below.
The T cell antigen receptor will falls under one of three categories, just like how Goldilocks tried three different bowls of porridge. T cell receptors that respond strongly to self-antigens found in the thymus send kill signals to the cell as a means to prevent autoimmunity. T cells that fail to react at all will die by neglect (T cells need periodic stimulation via their receptor to live). Cells that respond only mildly will be positively selected and graduate to bonafide T cell status. So, the thymus and T cells are the main reason the immune system knows what’s us and what isn’t. Now that we have a functioning T cell, let’s look a bit closer.
Bonafide T cells are divided into two major classes based on their surface protein expression of molecules called CD4 and CD8. Most mature T cells express one or the other and never both. Once a T cell decides, based on complex interactions with nurse cells in the thymus, it loses the ability to produce the other. Immunologists refer to this as restriction, a complicated series of molecular events that further train the T cell for its job of protecting the body. Restriction is also a leading cause of transplanted organ rejection.
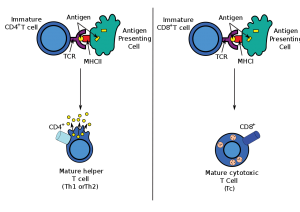 The important thing to remember is CD4 T cells are the “helpers” and CD8 T cells are the “killers”. T helper cells help B cells develop strong antibodies that can bind up and eliminate viruses and bacteria. Without proper T cell help, B cell responses stall and are highly inefficient. For example, children with DiGeorge Syndrome (a condition where patients lack a thymus completely) lack T cell help but have fully functional B cells. These patients will succumb to infection by 2 or 3 years of age without proper precaution and treatment. Killer T cells, also called cytotoxic T cells, are exactly what their name implies – licensed killers that will tear apart cells that show signs of infection. An example of this is hepatitis A, a viral infection of the liver. In hepatitis A, the CD8 killer T cells are unleashed on infected liver cells, killing them all. The temporary liver damage seen in hepatitis patients is partly due to the immune response against the virus.
The important thing to remember is CD4 T cells are the “helpers” and CD8 T cells are the “killers”. T helper cells help B cells develop strong antibodies that can bind up and eliminate viruses and bacteria. Without proper T cell help, B cell responses stall and are highly inefficient. For example, children with DiGeorge Syndrome (a condition where patients lack a thymus completely) lack T cell help but have fully functional B cells. These patients will succumb to infection by 2 or 3 years of age without proper precaution and treatment. Killer T cells, also called cytotoxic T cells, are exactly what their name implies – licensed killers that will tear apart cells that show signs of infection. An example of this is hepatitis A, a viral infection of the liver. In hepatitis A, the CD8 killer T cells are unleashed on infected liver cells, killing them all. The temporary liver damage seen in hepatitis patients is partly due to the immune response against the virus.
Immunology is a lot of fun because it’s a huge puzzle and we don’t have all the pieces yet. T cells are a perfect example of the beautiful complexity of how we think our immune system works. So be good to your thymus and long live the T cells!
Edited by Zoe Terwilliger and Rami Major
